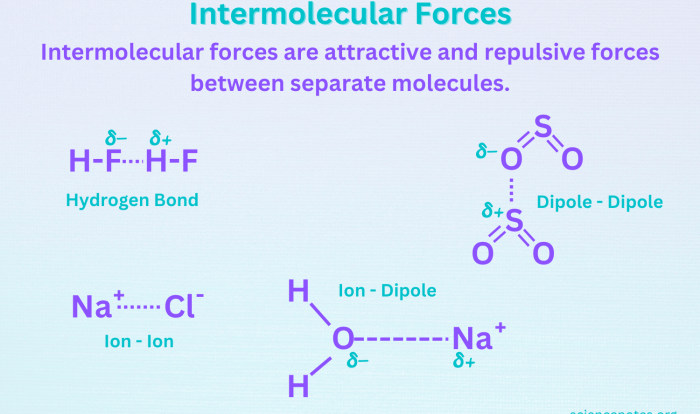
Naming and covalent compounds worksheet answers provide a valuable resource for students to enhance their understanding of chemical nomenclature and the properties of covalent compounds. This guide delves into the intricacies of naming binary covalent compounds, offering clear explanations and practical examples to facilitate comprehension.
The worksheet provided in this guide comprises a series of questions designed to test students’ grasp of covalent compound nomenclature. A comprehensive table is included to organize the answers, ensuring clarity and ease of reference.
Introduction
This worksheet aims to enhance your understanding of naming and covalent compounds, essential concepts in chemistry. Mastering these concepts will enable you to accurately describe and identify covalent compounds, a fundamental aspect of chemical nomenclature.
Naming Covalent Compounds
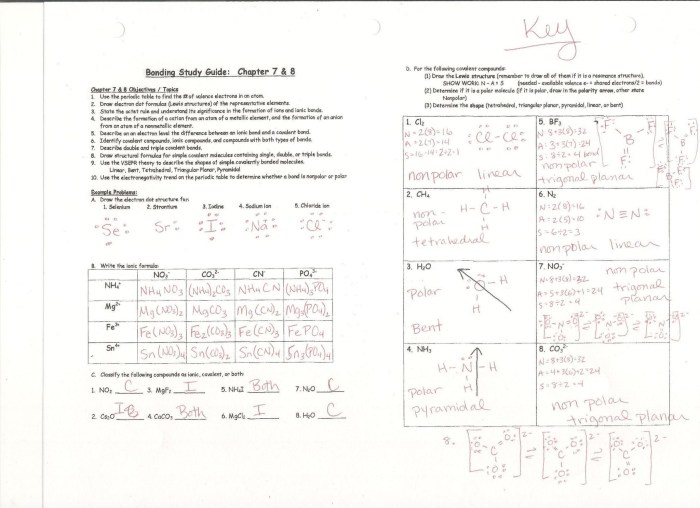
Binary covalent compounds are composed of two non-metallic elements. To name these compounds, follow these rules:
- Use the root name of the first element.
- Use the suffix “-ide” for the second element.
- Add prefixes to indicate the number of atoms of each element.
For example, the compound composed of one carbon atom and two oxygen atoms is named carbon dioxide.
Covalent Compounds Worksheet
Complete the following worksheet to practice naming covalent compounds:
- Name the compound: Cl 2O
- Write the formula for the compound: sulfur dioxide
- Name the compound: N 2O 5
- Write the formula for the compound: carbon monoxide
Worksheet Answers: Naming And Covalent Compounds Worksheet Answers
The answers to the worksheet questions are as follows:
- Chlorine monoxide
- SO2
- Dinitrogen pentoxide
- CO
Top FAQs
What is the purpose of naming covalent compounds?
Naming covalent compounds allows us to identify and describe these compounds using a systematic and standardized nomenclature system, facilitating communication and understanding among chemists.
How are binary covalent compounds named?
Binary covalent compounds are named using the prefixes mono-, di-, tri-, tetra-, etc. to indicate the number of atoms of each element present in the compound. The first element is named using the root of its element name, while the second element is named using the root of its element name followed by the suffix -ide.