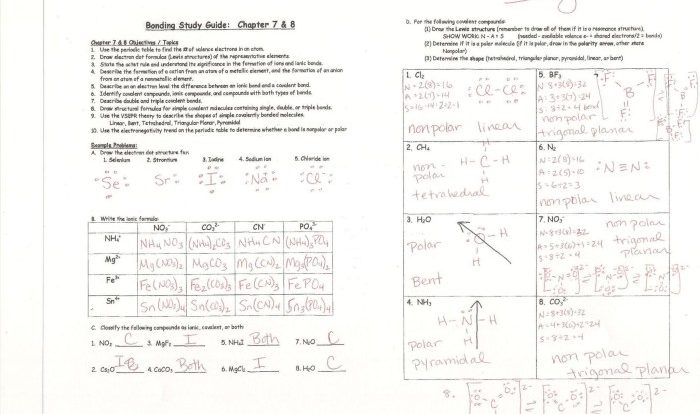
The types of intermolecular forces worksheet delves into the fascinating world of molecular interactions, revealing the fundamental forces that govern the properties and behavior of substances. These forces, including hydrogen bonding, dipole-dipole forces, and London dispersion forces, play a crucial role in shaping the physical and chemical properties of matter.
As we explore the nuances of intermolecular forces, we will uncover their profound impact on the properties of substances, ranging from their melting and boiling points to their solubility and reactivity. By understanding these forces, we gain insights into the behavior of molecules and their interactions with each other, paving the way for advancements in fields such as chemistry, biology, and materials science.
Types of Intermolecular Forces: Types Of Intermolecular Forces Worksheet
Intermolecular forces (IMFs) are attractive forces that exist between molecules and determine their physical properties. These forces are weaker than the intramolecular forces that hold atoms together within a molecule.
Hydrogen Bonding
Hydrogen bonding is the strongest type of IMF and occurs when a hydrogen atom is bonded to a highly electronegative atom (such as N, O, or F) and is also attracted to another electronegative atom.
Dipole-Dipole Forces
Dipole-dipole forces are IMFs that occur between polar molecules, which have a permanent dipole moment due to uneven distribution of electrons. These forces are weaker than hydrogen bonding but stronger than London dispersion forces.
London Dispersion Forces
London dispersion forces are the weakest type of IMF and occur between all molecules, regardless of their polarity. These forces arise from the temporary fluctuations in electron distribution, creating instantaneous dipoles that can interact with other molecules.
Properties of Substances with Different Intermolecular Forces

The strength of IMFs greatly affects the physical properties of substances. Substances with strong IMFs, such as water, have higher melting and boiling points, higher enthalpy of fusion and vaporization, and are generally denser and less volatile.
Examples
- Strong IMFs (hydrogen bonding):Water (H 2O), alcohols, and ammonia (NH 3)
- Weak IMFs (London dispersion forces):Methane (CH 4), ethane (C 2H 6), and hexane (C 6H 14)
Applications of Intermolecular Forces
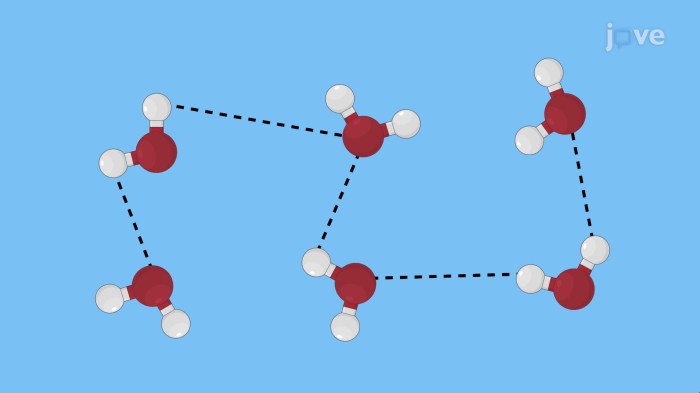
IMFs play a crucial role in various fields, including:
Chemistry
- Determining the solubility and reactivity of compounds
- Explaining the behavior of solvents and solutes
Biology
- Maintaining the structure and function of proteins and DNA
- Facilitating biological processes such as cell division and recognition
Materials Science
- Designing materials with specific properties, such as strength, flexibility, and thermal stability
- Developing new technologies, such as nanomaterials and self-healing materials
Measuring Intermolecular Forces
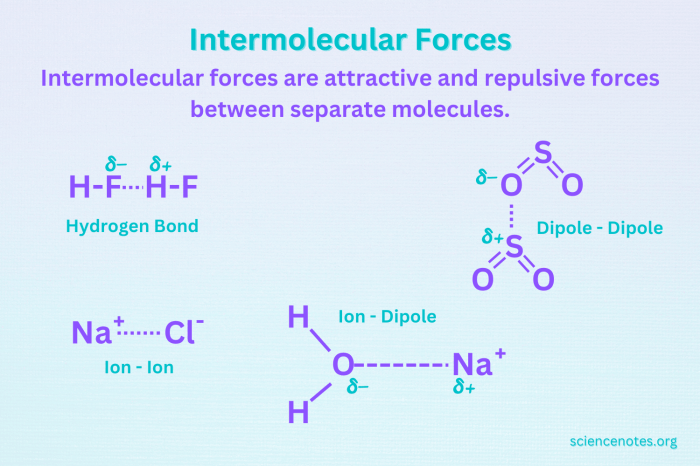
The strength of IMFs can be measured using various experimental techniques, including:
Vapor Pressure Measurement, Types of intermolecular forces worksheet
By measuring the vapor pressure of a liquid, the enthalpy of vaporization can be determined, which is related to the strength of IMFs.
Boiling Point Measurement
The boiling point of a liquid is also related to the strength of IMFs. Liquids with stronger IMFs have higher boiling points.
Viscosity Measurement
Viscosity is a measure of the resistance of a fluid to flow. Liquids with stronger IMFs have higher viscosities.
Frequently Asked Questions
What are the three main types of intermolecular forces?
The three main types of intermolecular forces are hydrogen bonding, dipole-dipole forces, and London dispersion forces.
How do intermolecular forces affect the properties of substances?
Intermolecular forces influence the physical properties of substances, such as their melting and boiling points, viscosity, and solubility.
What are some real-world applications of intermolecular forces?
Intermolecular forces find applications in various fields, including the design of new materials, drug development, and understanding biological processes.