The Alkene Addition Reactions Cheat Sheet is an invaluable resource for organic chemists, providing a concise and clear overview of the fundamental concepts and applications of alkene addition reactions. This cheat sheet covers the mechanisms, regioselectivity, and applications of electrophilic, nucleophilic, radical, and pericyclic addition reactions, empowering chemists with a comprehensive understanding of this essential topic.
Throughout this cheat sheet, we will explore the intricacies of alkene addition reactions, unraveling their mechanisms and regioselectivity. We will delve into the practical applications of these reactions in organic synthesis, showcasing their versatility and significance in the construction of complex molecules.
Introduction to Alkene Addition Reactions: Alkene Addition Reactions Cheat Sheet

Alkene addition reactions are a fundamental class of organic reactions that involve the addition of a new atom or group of atoms across the double bond of an alkene. These reactions are widely used in organic synthesis to construct complex molecules with specific functional groups.
The general mechanism of alkene addition reactions involves the nucleophilic attack of an electron-rich species (nucleophile) on the electrophilic carbon atom of the double bond, followed by the addition of an electrophile (electron-poor species) to the other carbon atom of the double bond.
This results in the formation of a new single bond between each carbon atom and the added atoms or groups.
Types of Alkene Addition Reactions
- Electrophilic addition reactions
- Nucleophilic addition reactions
- Radical addition reactions
- Pericyclic addition reactions
Electrophilic Addition Reactions

Electrophilic addition reactions are characterized by the addition of an electrophile to the double bond of an alkene. The electrophile is typically a positively charged ion or a molecule with a strong electrophilic group, such as a carbonyl group or a nitro group.
The mechanism of electrophilic addition reactions involves the formation of a carbocation intermediate, which is then attacked by a nucleophile to form the final product. The regioselectivity of electrophilic addition reactions is determined by the stability of the carbocation intermediate.
Examples of Electrophilic Addition Reactions
- Addition of hydrogen halides (HX)
- Addition of water
- Addition of alcohols
- Addition of carboxylic acids
Nucleophilic Addition Reactions
Nucleophilic addition reactions are characterized by the addition of a nucleophile to the double bond of an alkene. The nucleophile is typically a negatively charged ion or a molecule with a lone pair of electrons, such as an amine or an alkoxide ion.
The mechanism of nucleophilic addition reactions involves the direct attack of the nucleophile on the double bond, resulting in the formation of a new single bond between the carbon atom and the nucleophile. The regioselectivity of nucleophilic addition reactions is determined by the steric and electronic effects of the substituents on the double bond.
Examples of Nucleophilic Addition Reactions
- Addition of Grignard reagents
- Addition of organolithium reagents
- Addition of cuprate reagents
- Addition of alkynes
Radical Addition Reactions
Radical addition reactions are characterized by the addition of a radical to the double bond of an alkene. The radical is typically generated by the homolytic cleavage of a peroxide or an azo compound.
The mechanism of radical addition reactions involves the formation of a radical intermediate, which then reacts with the double bond to form a new single bond between the carbon atom and the radical. The regioselectivity of radical addition reactions is determined by the stability of the radical intermediate.
Examples of Radical Addition Reactions
- Addition of hydrogen atoms
- Addition of alkyl radicals
- Addition of aryl radicals
- Addition of vinyl radicals
Pericyclic Addition Reactions

Pericyclic addition reactions are characterized by the concerted addition of two or more atoms or groups across the double bond of an alkene. These reactions are typically catalyzed by a metal complex.
The mechanism of pericyclic addition reactions involves the formation of a cyclic transition state, in which the double bond of the alkene and the atoms or groups being added are all aligned in a specific orientation. The regioselectivity of pericyclic addition reactions is determined by the symmetry of the transition state.
Examples of Pericyclic Addition Reactions
- Diels-Alder reaction
- Ene reaction
- Cycloaddition reactions
- Electrocyclic reactions
Applications of Alkene Addition Reactions
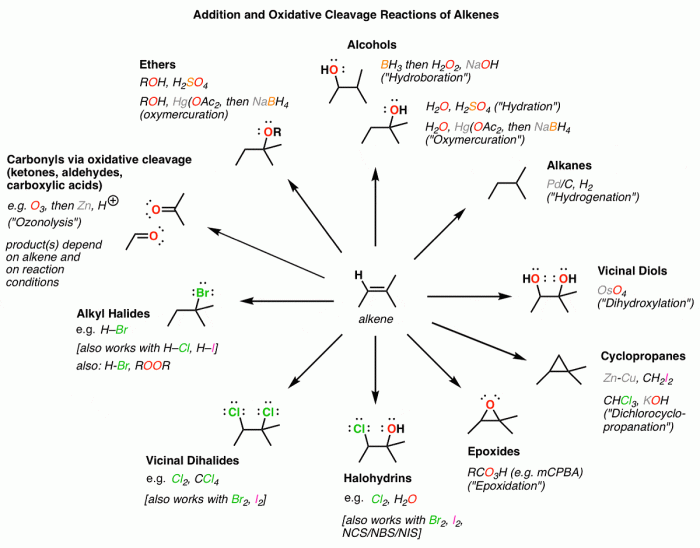
Alkene addition reactions are widely used in organic synthesis to construct complex molecules with specific functional groups. These reactions are particularly useful for the synthesis of alkenes, alcohols, ethers, and ketones.
Examples of the Use of Alkene Addition Reactions in the Synthesis of Complex Molecules, Alkene addition reactions cheat sheet
- Synthesis of ibuprofen (a non-steroidal anti-inflammatory drug)
- Synthesis of vitamin A (a fat-soluble vitamin)
- Synthesis of cholesterol (a steroid)
- Synthesis of rubber (a polymer)
Answers to Common Questions
What are alkene addition reactions?
Alkene addition reactions are a class of organic reactions in which a double bond between two carbon atoms in an alkene is broken and new bonds are formed with other atoms or groups of atoms, resulting in the addition of new functional groups to the alkene.
What are the different types of alkene addition reactions?
There are four main types of alkene addition reactions: electrophilic addition, nucleophilic addition, radical addition, and pericyclic addition.
What is the regioselectivity of alkene addition reactions?
The regioselectivity of an alkene addition reaction refers to the preference for the formation of one product over another based on the regiochemistry of the reaction. Regioselectivity is influenced by various factors, including the nature of the reactants, the reaction conditions, and the mechanism of the reaction.